
Introduction:
Water is the wellspring of life. It is a universal solvent; many types of salts dissolve in water. It is the most important liquid in the world for maintaining the plant and animal life. It fills lakes, streams, the vast oceans, and flows under the ground. Water is a remarkable chemical, an excellent solvent capable of dissolving, to varying degrees, almost anything with which it comes in contact. Water picks up suspended matter as it runs across the ground and absorbs gases from the atmosphere. Impurities in the water come from various sources.
There are different sources of water namely
Common impurities in water:
Water Quality:
Rapid industrialization, unplanned resources utilization and poor environmental management have affected the quality of water. Water quality is measured how it is suited for particular purpose for human needsand industrial purposes. With the ever increasing TDS content in raw water and treated effluent RO has been emerged as most economical technology.
| Water Resources | Definition |
| Fresh Water | Having Low salt concentration |
| Brackish Water | Salinity is more compared to fresh water less compared to sea water |
| Sea Water | Water from sea or ocean . Comparatively it has very high salinity |
| Waste Water | It is the domestic waste water, Community waste water, industrial waste water |
Water Quality Index:
To analyze the water quality there are some Water Quality test there are some factors to be considered, they are
 Temperature Temperature |
 Nitrate Nitrate |
 pH pH |
 Phosphate Phosphate |
 Chloride & Salinity Chloride & Salinity |
 Calcium & Water Hardness Calcium & Water Hardness |
 Dissolved Oxygen Dissolved Oxygen |
 Ammonium ion Ammonium ion |
 Turbidity Turbidity |
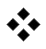 TDS TDS |
| Water Quality Characteristic & Measurements: | ||
| Hardness | It depends on the amount of dissolved calcium and magnesium present, measured in terms of calcium carbonate (CaCO3) | Soft 0-20 ppm
Moderately soft 20-40 ppm Moderately hard 40-80 ppm Hard 80-120 ppm Very hard >120 ppm |
| Total dissolved solids (salts) | Measurement of dissolved minerals including sodium, calcium, magnesium, potassium, chloride, fluoride, sulphide, carbonate, and bicarbonate. | Surface Water : 100 – 2000 ppm
Brackish Water : 2000 – 10000 ppm Sea water : 10000 – 35000 up to 55000 ppm |
| pH | Measurement of acidity. pH of Drinking water is 6 – 8.5. | Ranges from 0 -14 , 7 is Neutral
<7 Acidity >7 Base. |
| Turbidity | It is a measure of relative clarity or cloudiness of water.it is measured in Nephelometric Turbidity Units. Heavy Water flow turbidity is high whereas in particles settled still water it is low. | Nephelometric Turbidity Units (NTU)
Surface Water – 1 NTU – 50 NTU Drinking Water – 0.5 NTU to 1.0 NTU |
| Dissolved Oxygen | To measures the oxygen dissolved in water by aquatic organism. | Depends on Temperature Ranges from 0 to 15ppm .Cold mountain Stream has 7 – 15 ppm
water closer to sea level – 2 to 11 ppm. |
| Phosphates | Excess Phosphates results in algal growth. | Above 0.1ppm stimulates the growth of algal. |
| Nitrate | Excess Nitrate will create a difficult condition to aquatic life. | Fresh Water – 0.1 to 4 ppm
Unpolluted waters – Less than 1 ppm Waste water – above 20ppm |
Note: *water qualities are measured as concentration in milligrams (a thousand of a gram) in one liter of water (mg/L).It can also be
described in “part per million”(ppm) 1 (ppm) = 1 (mg/L).
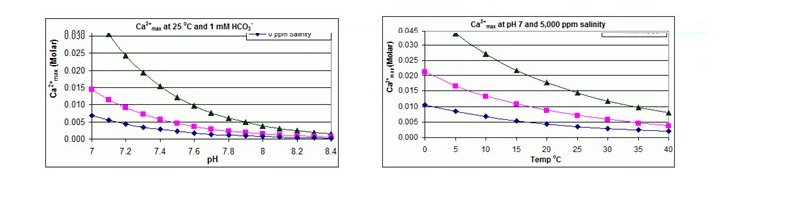
Calcium Carbonate:It is well recognized that calcium carbonates (CaCO3) is one of the main components of scale that is commonly encountered in chemical and related industries. The calcium carbonate scale often grows extensively, causing major operational difficulties.The temperature and pH values affect the kinetics of crystal formation.
The solubility of Calcium Carbonate is typically limited to a LSI (Langlier Saturation Index) value of positive 1.8 to 2.5.Calcium Carbonate solubility is measured using LSI (Langlier Saturation Index) forbrackish waters or SDSI (Stiff and Davis Saturation Index) for seawaters and is lower with increasing temperature and increasing pH.
The solubility product for CaCO3 and the dissociation constants for the DIC (Dissolved Inorganic Carbon) species are all substantially affected by temperature and salinity with the overall effect that Ca2+max increases from fresh to salt water, and decreases with rising temperature, pH, or added bicarbonate level, as illustrated in the accompanying graphs.
| Measurement Index For Water Quality | ||
| LSI –
Langelier saturation index / Langelier Stability index |
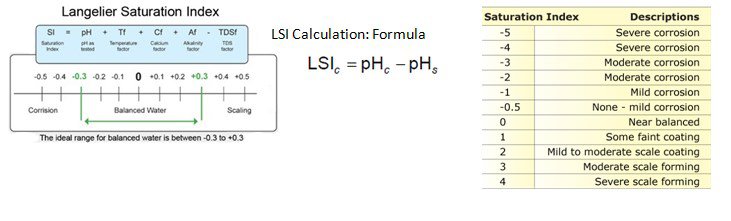
It predict the calcium carbonate stability in water whether it will dissolve or precipitate or will remain equilibrium. It shows the scaling potential of CaCO3 | For LSI > 0, precipitate of CaCO3.
For LSI = 0, Equilibrium state, CaCO3 is neither precipitated nor dissolved. For LSI < 0, CaCO3is dissolved LSI = pH – pHs (TDS <10,000 ppm) |

| Measurement Index | ||
| SDI –
Silt Density Index |
It is used to measure the fouling potential of suspended solids on RO membrane. Surface or seawater having SDI up to 200, requiring flocculation, coagulation, and more high tech filtration before RO treatment. | SDI <1 = No fouling for several years
SDI <3 = No fouling for months SDI 3-5 = fouling is likely SDI >5 – Unacceptable, pretreatment is needed |
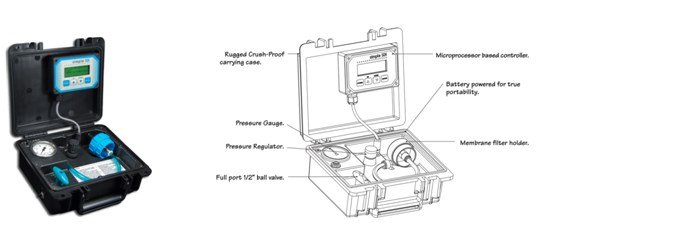
To calculate SDI follow these steps:
Step 1: Measure the time required to filter a fixed volume of water eg:- 500ml through a standard
0.45μm pore size microfiltration membrane at a constant pressure of 30 psi (2.07 bar). Record this as
Ti, or T initial.
Step 2: Take additional time measurements, normally after 5, 10 and 15 minutes (after silt build up)
Step 3: Calculate the Plugging Factor after 5, 10 and 15 minutes as follows:
PF5 = (1-Ti /T5)*100
PF10 = (1-Ti /T10)*100
PF15 = (1-Ti /T15)*100
Step 4: The SDI value is then determined at each interval as SDI = PF/T.
| Measurement Index | ||
| S & DSI [Stiff & Davis Saturation Index] | It helps to predict the scaling and corrosion potential of high TDS sea water based on the saturation of CaCO3.
S & DSI is used for high TDS ranges (>10,000 ppm). It is for sea water. S&DSI = pH – pHs (TDS >10,000 ppm) |
If TDS is above 10000 ppm S&DSI is used |